Patentability of Genetic Material – Australian Rules
Patentability of Genetic Material – Australian Rules
Patentability of Genetic Material – Australian Rules
25 Mai 2016
Aptean Staff Writer
After the United States Supreme Court ruling in the Association for Molecular Pathology v. Myriad Genetics in June of 2013, the industry scurried. The Court ruled that naturally occurring DNA is not patent eligible even if isolated, but cDNA or “complementary DNA” is because it is not naturally occurring but rather a product of the laboratory scientist even though it is exactly the same nucleic acid information.
In fact, however, cDNA is naturally occurring as many viruses convert their viral RNA into mRNA through cDNA: viral RNA → cDNA → mRNA. Unfortunately, the U.S. Supreme Court ruling, based in a lack of understanding of the science, created the broadest possible interpretation of isolated DNA and cDNA. This ruling forced the USPTO to create even more complex guidelines to determine whether inventions were patent eligible and opened the door for dozens of lawsuits on the eligibility of claims formerly granted pre-Myriad.
Australia recently undertook the same challenge – in this case the Australian High Court took on D’Arcy v Myriad Genetics and ruled similarly that Myriad’s claims relating to isolated BRCA1 nucleic acid were not patent eligible subject matter. However, in Australia’s case, the clear, scientifically grounded, well-reasoned ruling enabled the Australian Patent Office to release narrow new guidelines that promote innovation by setting clear rules.
In particular, they note that any naturally occurring subject matter which merely claims genetic information, be it isolated DNA, RNA, whether human or non-human, coding or non-coding is excluded as patentable. In addition, they point out that any man-made constructs that do nothing more than replicate genetic information of a naturally occurring organism are also excluded, including cDNAs, probes, etc. They clarify that this excludes man-made constructs that are not naturally occurring, be they chimeric DNA or novel antibody sequences.
In addition to clear specifications of what is excluded, the High Court also clarified what can be considered as patent eligible. These include:
Recombinant or isolated proteins
Pharmaceuticals and other chemical substances
Methods of treatment
Methods of applying herbicides
Applications of computer technology
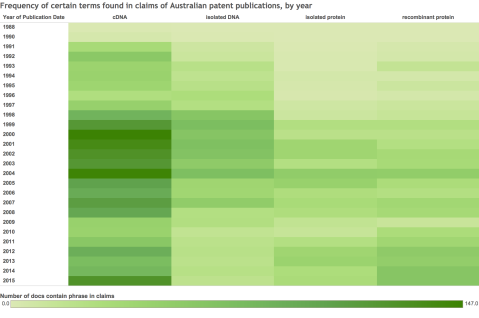
Wanting to visualize trends in patenting and any recent effects of these changes, we generated this heat map with a combination of LifeQuest and GenomeQuest data. It depicts all Australian grants and applications that contain any one of the phrases cDNA, isolated DNA, isolated protein or recombinant protein occurring in the claims.
Note how the High Court ruling affects a significant number of documents that refer to cDNAs during the ten year span from 1998 to 2008, and how the claimed subject matter for recombinant proteins continues to grow in recent years. The significant increase in 2015 of the term cDNA in claims made of of a large number of new applications with rewritten claims that still refer to cDNAs but are directed toward methods for using them in diagnostic and therapeutic contexts.
The message is clear: with clear guidelines for subject matter provided by the Australian High Court and Patent Office, innovators were quick to see the writing on the wall and reformulate their IP in Australia.
With its extensive data coverage (over 500 million sequences), powerful search tools and user-friendly functionality, Aptean GenomeQuest is the obvious choice for searching the entire sequence domain, both patent and non-patent.
Avoid the pitfalls of using free solutions for IP sequence searching. Download our RFP template or start a free trial today!
Related Content


Bereit für die Auswahl Ihrer IP-Sequenzsuche
Nutzen Sie unsere kostenlose RFP-Vorlage (Request for Proposal), um die richtige IP-Sequenzsuchlösung für Ihr Unternehmen zu finden.